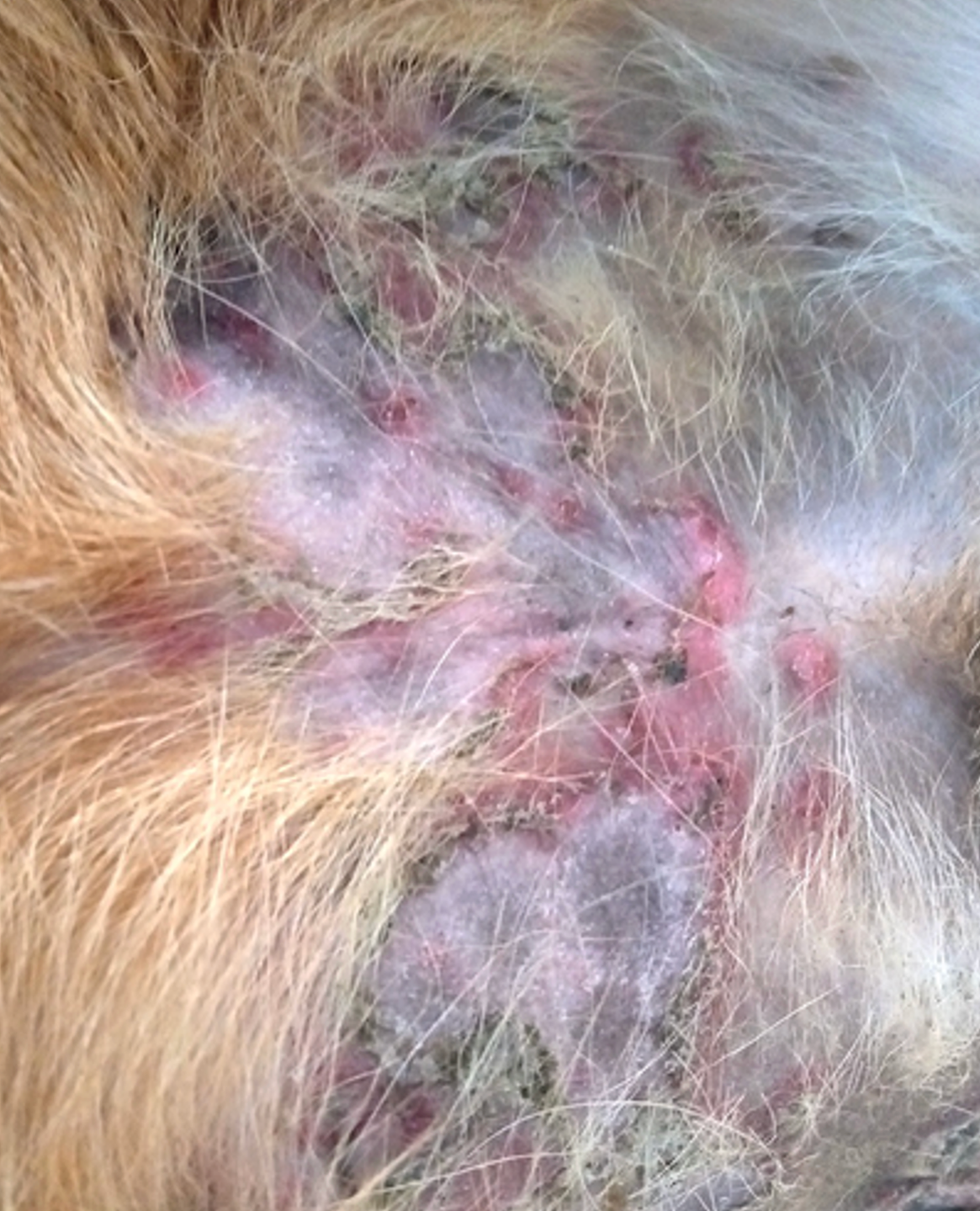
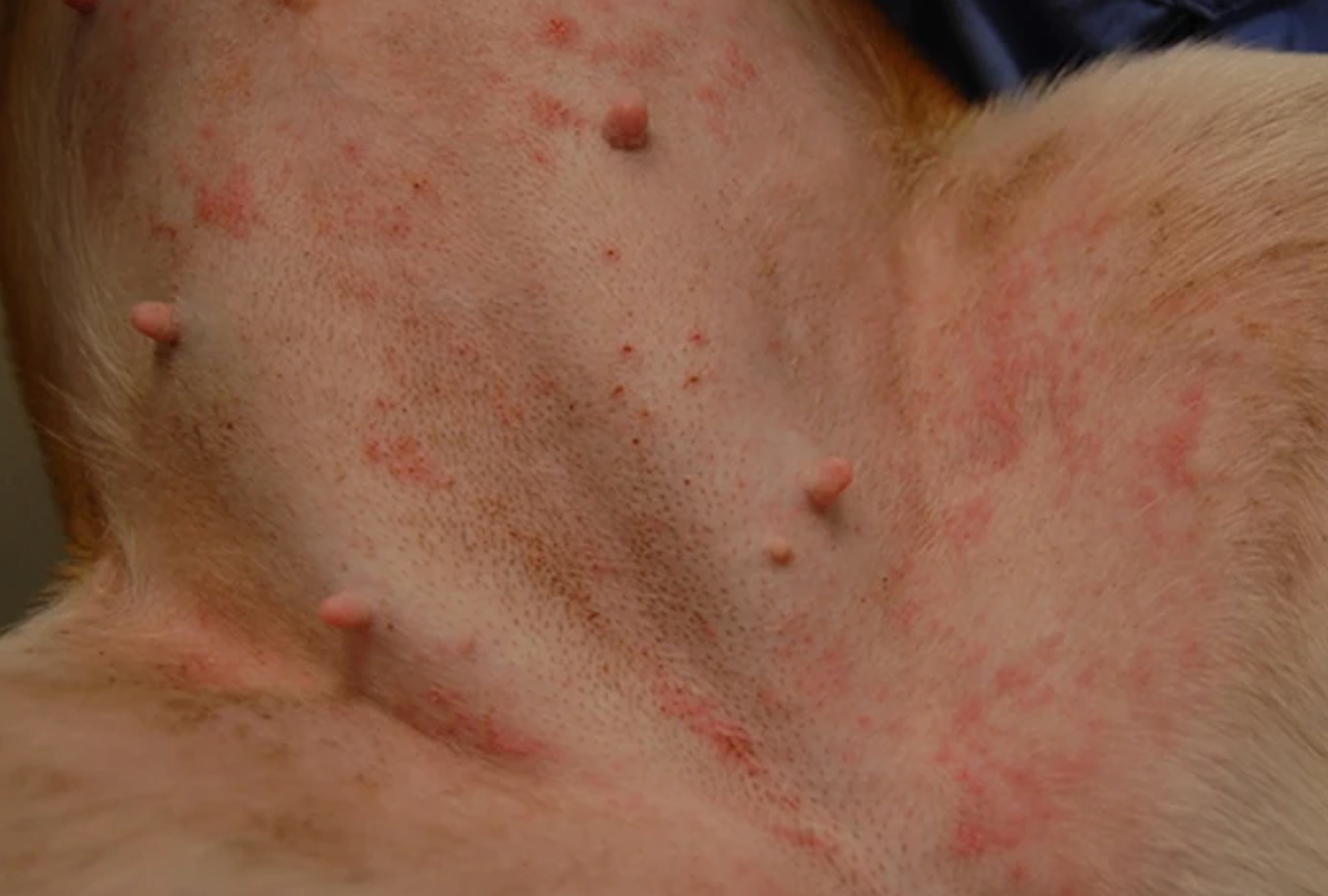
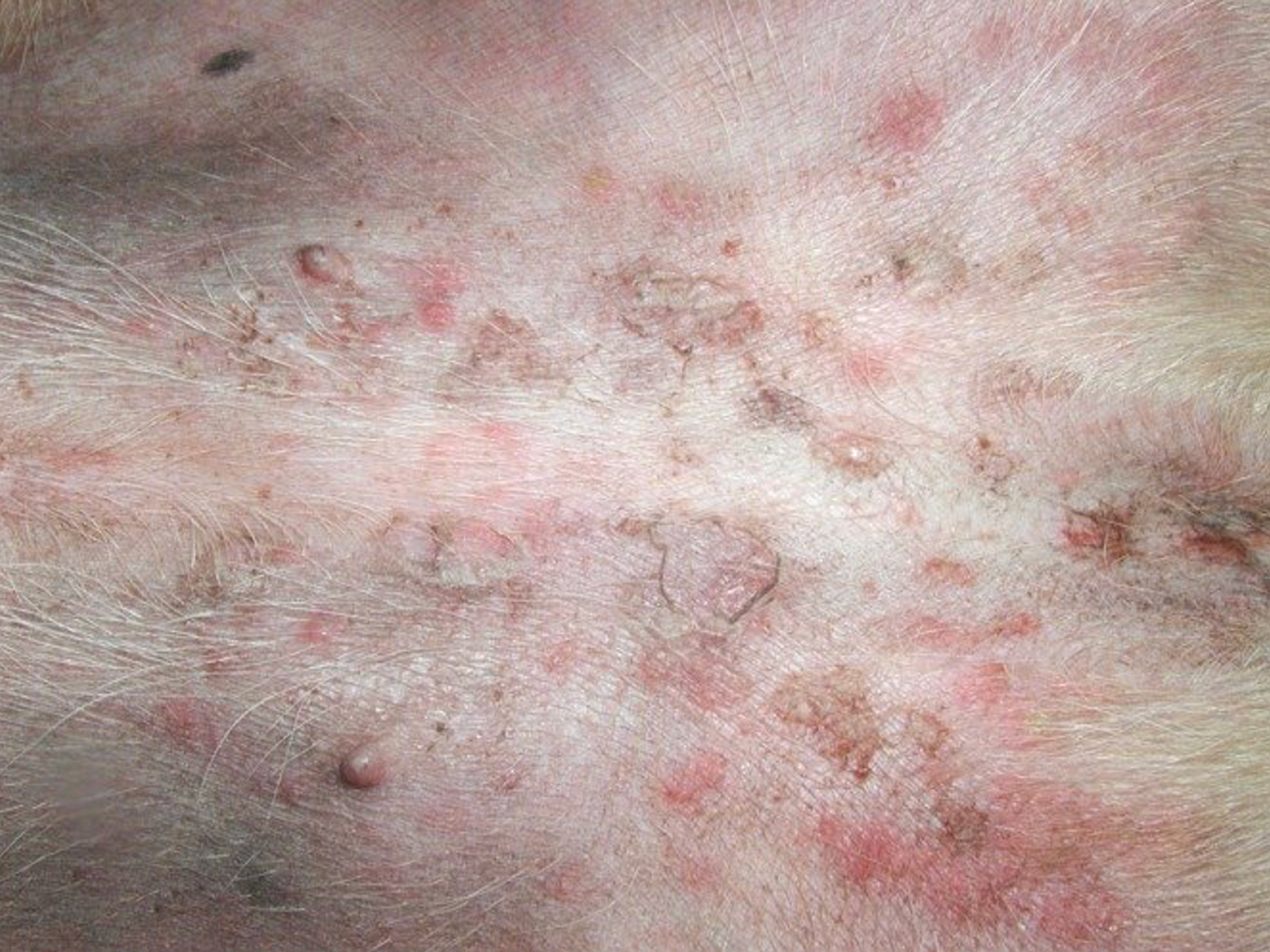
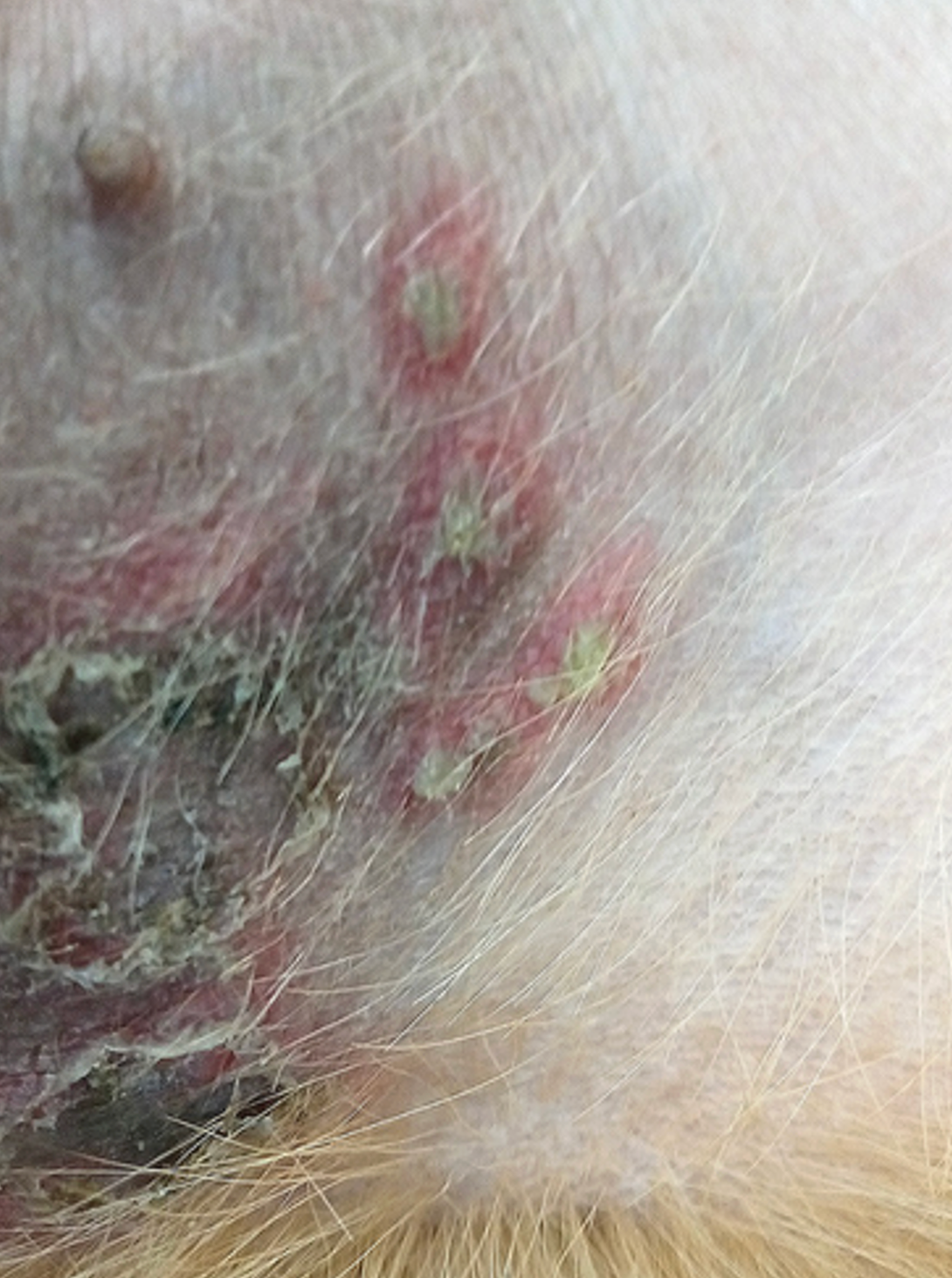
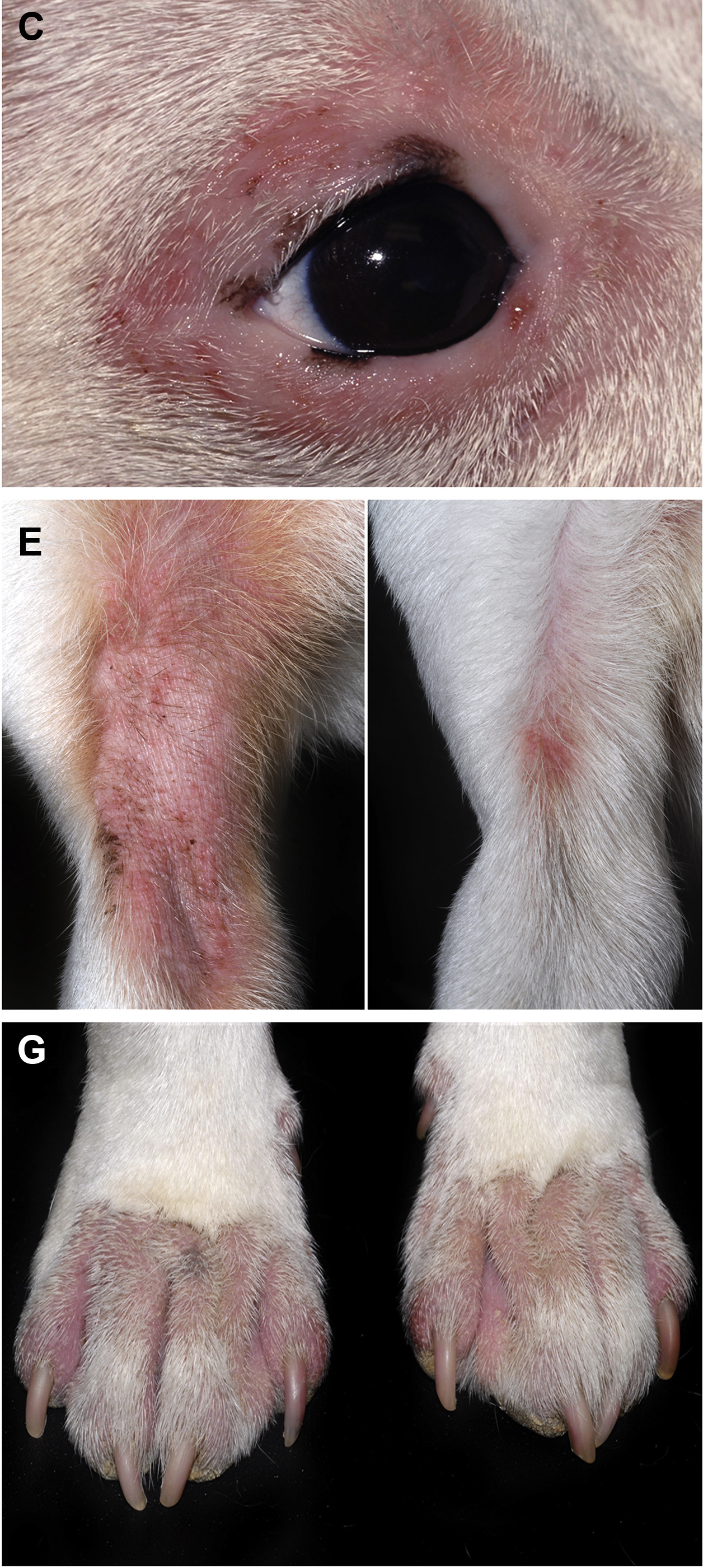
For decades following the recognition of atopic dermatitis (AD) as a common allergic skin disease of dogs, its treatment relied mainly on the use of oral — and more often than not injectable — glucocorticoids. When the “time was right”, allergen immunotherapy (AIT) was used to reduce the need for glucocorticoids because of their ubiquitous adverse effects. It was only in 2004 that the calcineurin inhibitor ciclosporin (Atopica, Elanco; Greenfield, IN, USA) became the first immunomodulator specifically approved for treatment of canine AD. In 2007, the hydrocortisone aceponate-containing spray Cortavance (Virbac; Carros, France) was the second pharmacological intervention approved for allergic skin diseases. More recently, in 2014, oclacitinib (Apoquel, Zoetis; Parsippany, NJ, USA) was the first-in-class Janus kinase (JAK) inhibitor available to treat AD in dogs, years before another JAKinib will be approved to treat the human disease homologue.1 Finally, 2017 saw the first monoclonal antibody (mAb) fully approved for a canine disease, the antiinterleukin (IL)-31 lokivetmab (Cytopoint, also from Zoetis) for treatment of canine AD.